In this post I will try to explain the difference between baking soda and baking powder and how they each work to rise baked goods.
Baking soda is pure Sodium Bicarbonate. Sodium Bicarbonate is alkaline in nature.
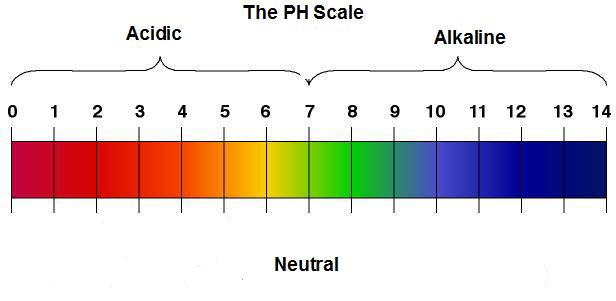
Maybe we should review the the ph scale before we go any further so that every one is on the same page. This is an image depicting the ph scale:
As you can see, 7 is neutral. Pure water has a ph level of 7.
Anything with a ph of less than 7 is acidic in nature. Each increment increases by ten fold. So, 6 is ten times more acidic than 7, and 5 is one hundred times more acidic than 7.
Any numbers higher than 7 are alkaline, also, increasing alkalinity in ten fold increments. So, 8 is ten times for alkaline than 7, and 9 is one hundred times more alkaline than 7, and so on and so on.

Sodium Bicarbonate has a ph of 9 and is an alkaline substance. It reacts with acids to produce carbon dioxide. Have you ever made a volcano with baking soda and vinegar? What happened when the baking soda and vinegar were combined? A lot of carbon dioxide was produces and caused the mixture to bubble so much that in bubbled right over the top of the "volcano," right?
Well, when we bake with sodium bicarbonate (baking soda) we use the same kind of reaction to rise our baked goods. A recipe using baking soda for leavening needs to have an acidic ingredient to cause the reaction. Things like buttermilk, vinegar, sour cream, yogurt, and even honey and molasses, are acidic and will cause baking soda to react. As soon as the acids and baking soda are combined with a liquid in a recipe they begin reacting and producing carbon dioxide. (Almost always the acid is a liquid so the reaction is usually instant.) The carbon dioxide starts to bubble to the surface and as it rises it carries the batter with it causing the end product to be light and fluffy.
Baking powder works similarly. The difference between baking powder and baking soda is that baking soda is pure sodium bicarbonate and baking powder is sodium bicarbonate combined with an acid and some corn starch. Baking powder can be used in recipes without acidic ingredients because it contains the acid it needs to react and produce carbon dioxide. It just needs to be combined with a liquid. The corn starch in baking powder helps to keep the acid and the sodium bicarbonate separate so that the humidity in the air does not cause them to start reacting.
If you are ever out of baking powder you can make your own if you have baking soda and cream of tartar. Cream of tartar is potassium bitartrate, which is an acid. To make baking powder from baking soda and cream of tartar; combine 1 teaspoon of baking soda and 2 teaspoons of cream of tartar for every tablespoon of baking powder.
Most store bought baking powders use two acids. One that reacts when liquid is added (monocalcium phosphate) and one that reacts when heated. This is what makes double acting baking powder, double acting. Most double acting baking powders use sodium aluminium sulfate as one of the acids because it reacts with sodium bicarbonate when heated. Aluminum is known to contribute to many health problems including Alzheimer's disease. Some manufactures produce non-aluminum, double acting baking powder. Instead of using sodium aluminium sulfate they use sodium acid pyrophosphate which contains no aluminum and also reacts at high temperatures.
I hope you have enjoyed this miniature chemistry lesson. Feel free to ask any questions you may have in the comments below and I will be happy to answer them!


This was interesting to read! :)
ReplyDeleteThat's the same type of baking pwdr that I use, I love it it that it doesn't have aluminum. Thanks for the post!
ReplyDelete